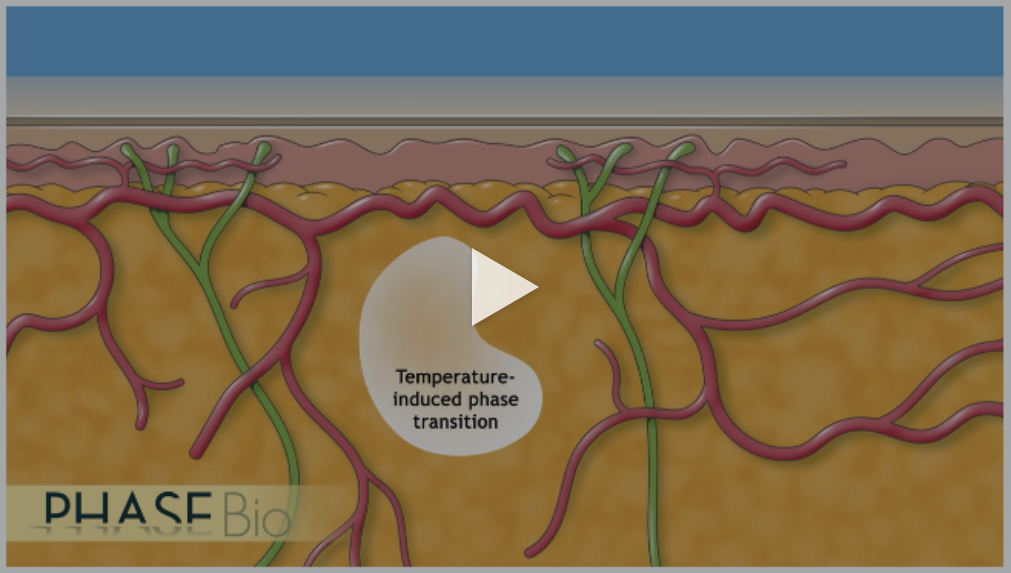
How It Works
ELP fusion proteins can undergo a temperature-dependent reversible phase transition. At lower temperatures ELP fusion proteins are completely soluble, while at warmer temperatures the ELP fusion proteins are in a gel-like state. This allows the ELP fusion proteins to be easily handled and administered subcutaneously using standard, fine-gauge needles and syringes. Once the ELP fusion protein is exposed to body heat, it forms a drug depot that slowly releases soluble ELP fusion proteins into the circulation. By modifying the amino acid sequence of the individual subunits and by varying the overall length of the ELP biopolymer, we can engineer our ELP fusion proteins to be released on timescales extending to a week or longer.
We produce our ELP-based products by engineering E. coli to produce a single protein comprising the active peptide or protein fused to the ELP biopolymer. This molecule is active as a fusion protein and does not require cleavage or release of the peptide. ELP fusion proteins are produced in the soluble fraction of E. coli, which allows for ease of scale up and purification.
PhaseBio product candidate pemziviptadil is engineered with an ELP biopolymer to provide once-weekly dosing. However, our ELP biopolymers can also be designed to provide faster or slower uptake from the injection site, enabling the development of convenient and patient-friendly dosing regimens.